Identify Each of the Following Substances as a Strong Electrolyte
Identify each of the following substances as strong electrolyte week electrolyte or nonelectrolyte. O nonelectrolyte O weak electrolyte O strong electrolyte c HNO3.
Solved Identify Each Of The Following Substances As A Strong Chegg Com
Identify each of the following substances as a.

. Add your answer and earn points. Click hereto get an answer to your question Classify the following substances under three headingsStrong electrolytes Weak electrolytes. Weak electrolyte a weak acid c KOH strong electrolyte a strong soluble base d FeOH 3 strong electrolyte an insoluble strong base.
A Ca3PO42 b MnOH2 c AgClO3 d K2S Write ionic and net ionic equations for the following reactions. NH42CO3 CaCl2 d. Identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte.
Next ammonium ion is a weak electrolyte. The passage of electricity through an electrolyte solution is caused by the movement of. Identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte.
Identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte. H 2 O Week electrolyte KCl Strong electrolyte HNO 3 Strong electrolyte BaOH 2 Strong electrolyte C 6 H 6 Nonelectrolyte 2. Draw the KCl scheme when dissolved in water.
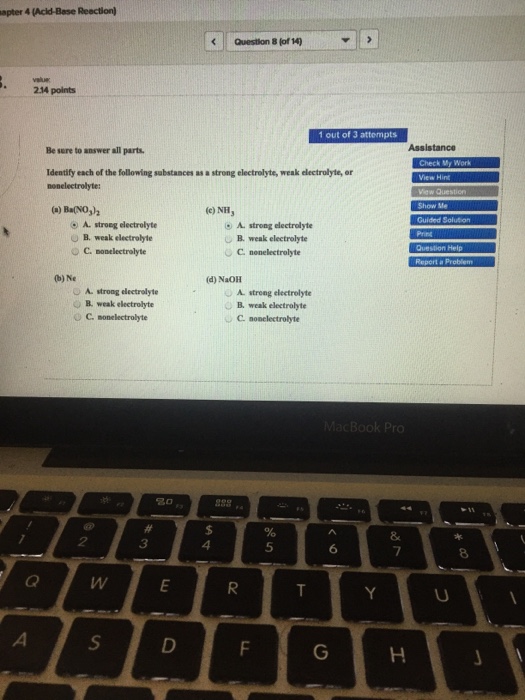
A BaNO 3 2 b Ne c NH 3 d NaOH. Find step-by-step Chemistry solutions and your answer to the following textbook question. A H2O b KCl c HNO3 d CH3COOH e C12H22O11.
Note that strong acids form strong electrolytes. Write ionic and net ionic equations for the following reactions. A BaNO32 O strong electrolyte O weak electrolyte O nonelectrolyte b Ne strong electrolyte weak electrolyte O nonelectrolyte C NH strong electrolyte weak electrolyte nonelectrolyte d NaOH strong electrolyte weak.
A Ba NO 3 2 b Ne c NH 3 d NaOH. 1 Define electrolyte and nonelectrolyte and identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte. Strong electrolytes conduct electricity very well.
Define electrolyte and nonelectrolyte and identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte. Examples of this would be vinegar and bleach which could be sodium hypochlorite or chlorine which are weakly dissociated. So lets talk about strong versus weak versus non electrolytes.
Join Login Class 12 Chemistry Electrochemistry Electrochemical Cells Classify the following substances under. Be predicting with these examples of bases are strong weak or non electrolyte. Identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte.
Shows the hydration for each ion. Identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte. Identify each of the following substances as strong electrolyte weak electrolyte or nonelectrolyte.
Answer to Identify each of the following substances as a strong electrolyte weak electrolyte or non electrolyte. NaNO3 KCl. So very um this industry is a non electrolyte because this is insoluble in water.
Identify each of the following substances as a strong electrolyte weak. A H2O b KCl c HNO3 d CH3COOH e C12H22O11 sucrose. And so an electrolyte is a substance that will conduct electricity.
Identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte. Identify each of the following substances as a strong electrolyte SE weak electrolyte WE or nonelectrolyte NE. Salt water baking soda NaHCO3 solution.
A weak electrolyte dissociates Less than 100. A H₂O bKCl c HNO₃ d HC₂H₃O₂ e C₁₂H₂₂O₁₁ a EXTREMELY weak b Strong c d e 913. BaCl2 ZnSO4 c.
Solve Study Textbooks Guides. A AgNO3aq Na2SO4aq b. Identify each of the following substances as a strong electrolyte weak electrolyteor nonelectrolyte a H 2 O b KClcHNO 3 d HC 2 H 3 O 2 e C 12 H 22 O 11 Step-by-step solution Step 1 of 4.
Identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte. Identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte. Recall that all ionic compounds are considered strong electrolytes because even if only a small amount of the compound dissolves in water the amount that does dissolve breaks apart to form Fe3 and OH- e CH 3 CH.
Neon is not electrolyte single. It does not fully associate water and sitting my drops. That is a strong electrolyte because it does fully associate.
A BaNO32 b Ne c NH3 d NaOH e. Step-by-step solution 100. A Ba NO32 B Ne C NH3 D NaOH amitathik9826 is waiting for your help.
Weak electrolytes do not completely dissociate in solution and hence have a low ionic yield. A strong electrolyte is one that dissociates or breaks apart 100. AgNO3 Na2SO4 b.
The strong electrolytes here are. Need help with chemistry. O nonelectrolyte O weak electrolyte O strong electrolyte b KCI.
A H2O b KCl c HNO3 d HC2H3O2 e C12H22O11 Characterize the following compounds as soluble or insoluble in water. Identify each of the following substances as a strong electrolyte weak electrolyte or nonelectrolyte. And the non electoral light does not disassociate.

Module 3 Connect Be Sure To Answer All Parts Identify Each Of The Following Substances As Homeworklib

Solved Identify Each Of The Following Substances As A Strong Chegg Com

Solved Be Sure To Answer All Parts Identify Each Of The Chegg Com
Comments
Post a Comment